Patented technologies for which Rebirthel has an exclusive license
(Summary)
Rebirthel has multiple patented technologies, and the following patents are three of particular importance. In all cases, Rebirthel has the exclusive license, which further allows us to sub-license to third parties. 1) TCR-iPSC method and 2) DP method have been patented in JP, US, EU, AU, and 3) TCR cassette method has been patented in Japan (as of November 2024).
As shown in the figure below, these three technologies provide a method to produce iPS cells that can be material cells and a method to induce differentiation of iPS cells into killer T cells. In particular, 1) TCR-iPSC method is the very basic technology for regenerated T cell therapy. We believe this TCR-iPSC method is a strong patent that covers a wide range of applications.
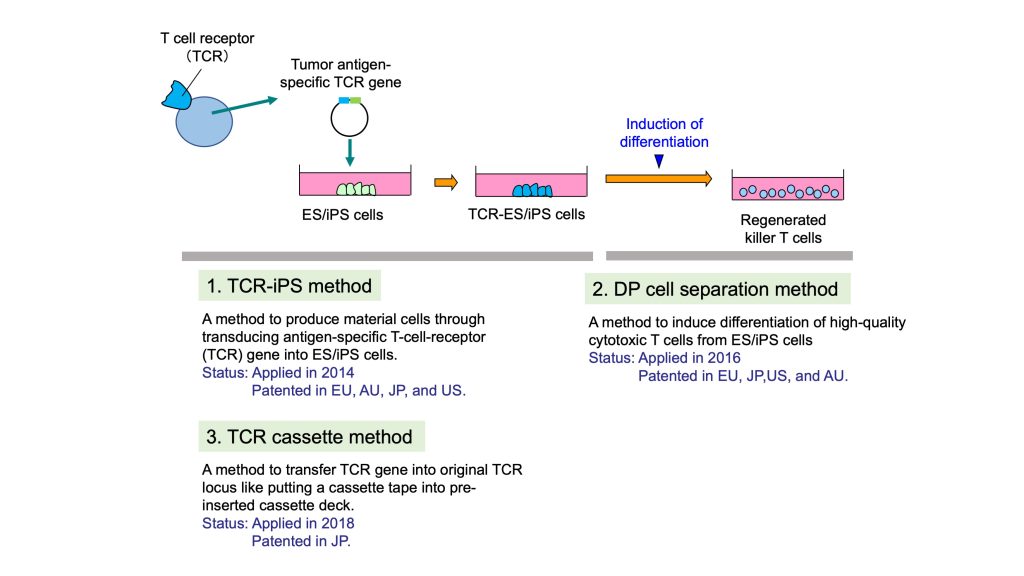
Each patent is explained in detail below.
1) TCR-iPS method
i) Title: PRODUCTION METHOD FOR PLURIPOTENT STEM CELLS HAVING ANTIGEN-SPECIFIC T CELL RECEPTOR GENE
ii) Application / Publication number: PCT/JP2015/070623 / WO 2016010154 A1
iii) Applicants: Hiroshi Kawamoto
iv) Inventors: Hiroshi Kawamoto, Kyoko Masuda, Takuya Maeda, Yoshimoto Katsura
v) Abstract
a) This is a method to produce T cells from T-cell-receptor (TCR) gene-transduced iPS cells. Patented in EU, AU, JP, and US. Under review in CN.
b) The fact that this technology has been globally patented indicates the absence of similar papers or patents and shows the uniqueness of Rebirthel’s strategy.
c) As indicated in the claim noted in the next section, this patent covers broad contents.
・As for TCR gene to be transferred, the claim covers not only TCR but also TCR gene.
・T cells made from TCR-iPS cells are not limited to killer T cells. The claim covers all types of T cells, including helper T cells, regulatory T cells, NKT cells, and T cells, etc.
・TCR gene transfer method is not limited. The claim covers any transducing methods using e.g. retroviruses, lentiviruses, or genome editing.

vi) The first item in the claim
1. An in vitro method for inducing T cells expressing a single T cell receptor for a cell-based immunotherapy, comprising the steps of:
(1) providing human induced pluripotent stem (iPS) cells;
(2) introducing re-arranged genes encoding an antigen specific T cell receptor into the iPS cells; and
(3) inducing T cells from the iPS cells obtained in step (2), thereby obtaining T cells expressing said antigen specific T cell receptor as the single T cell receptor,
wherein none of the genes encoding Rag 1 and Rag 2 in the iPS cells is knocked out, and wherein the iPS cells were induced from a somatic cell other than a T cell.
vii)Status
- July 2014: Initial application filed in Japan
- 2018: PCT application into the national phase in JP, US, EU AU, and CN.
- December 2019: Patented in EU
- July 2021: Patented in AU
- September 2021: Patented in JP
- August 2024: Patented in US
- Under review in CN.
viii) Additional notes
a) Rebirthel can license this patented technology unless the target antigen competes with Rebirthel or Rebirthel’s licensee.
b) An academic paper showing that the TCR-iPSC method is realizable was published in Molecular Therapy – Methods & Clinical Development, 19:250, 2020. In this article, it was demonstrated that killer T cells regenerated by using the TCR-iPSC method are equivalent to killer T cells regenerated by using the T-iPSC method, which utilizes iPSCs produced from T cells (T-iPSCs) as starting material. Subsequently, the effectiveness of killer T cells regenerated with TCR-iPSC method was confirmed by animal models (Kashima er al., iScience, 23:100998. 2020). In this article, human renal cell cancer was xenografted to a immunodeficient mouse, and the inhibitory effect on tumor growth was demonstrated when the regenerated killer T cells were administered to the mouse.
c) Two patent oppositions were raised when it was patented in Japan. On July 14, 2022, the Japan Patent Office rejected both oppositions, and the registration of this patent was finalized in Japan.
d) Though this is the result of research Professor Hiroshi Kawamoto conducted at Kyoto University, the applicant of this patent is only Hiroshi Kawamoto himself. Kyoto University filed the priority application in Japan in 2014. However, Kyoto University abandoned it at the time of PCT application in 2015, and the patent right was returned to Professor Kawamoto who is the first inventor. Professor Kawamoto personally filed PCT application as an applicant.
2) DP cell separation method (Method to induce high killing ability
Cytotoxic T lymphocytes (CTLs))
i) Title: METHOD FOR INDUCING CD8+ T CELLS
ii) Application / Publication number: PCT/JP2017/015358 / WO 2017/179720
iii) Applicants: Kyoto University
iv) Inventors: Hiroshi Kawamoto, Kyoko Masuda, Takuya Maeda
v) Abstract
This is a method to induce differentiation of high-quality cytotoxic (killer) T cells from iPS cells. In the process of differentiation of T cells from iPS cells, CD4+ CD8+ (double positive: DP) immature T cells are induced at a certain point. Using conventional methods wherein full population was stimulated, only CD8aa type cells can be obtained, which are weak in antigen-specific response. In this method, DP cells are isolated from other cells and stimulated. It enables the induction of highly cytotoxic CD8ab killer T cells. It was patented in EU in July 2020, in JP in September 2021, in US in August 2022, in AU in December 2022.

vi) Status
- April 2016: Initial application filed in Japan
- 2018: PCT application into the national phase in JP, US, EU, and AU
- July 2020: Patented in EU
- September 2021: Patented in JP
- August 2022: Patented in US
- December 2022: Patented in AU
vii) Additional notes
a) Rebirthel can license this patented technology unless the target antigen competes with Rebirthel or Rebirthel’s licensee.
b) An academic paper showing the efficacy and safety of DP cell separation method was published in Cancer Research, 76:6839, 2016.
c) DP cell separation method can be applied to the method in which iPS cells are transduced with CAR gene and then T cells are induced from these iPS cells.
3) TCR cassette method
i) Title: METHOD FOR PRODUCING FOREIGN ANTIGEN RECEPTOR GENE-INTRODUCED CELL
ii) Application / Publication number: PCT/JP2019/029537 / WO 2020/022512Y
iii) Applicants: Kyoto University, Shiga University of Medical Science
iv) Inventors: Hiroshi Kawamoto, Kyoko Masuda, Seiji Nagano, Yasutoshi Agata, Koji Terada
v) Abstract
This is the method evolved from TCR-iPSC method. In TCR-iPSC method, gene delivery was carried out by a general gene transfer method such as the one using lentivirus. Therefore, TCR is randomly integrated into the genome. This gene delivery leads to the following issues: 1) risk of genomic damage and 2) unstable expression. With this TCR cassette method, TCR gene is transferred into original TCR locus so that endogenous enhancer and promoter can control gene expression. Moreover, in this method, a cassette deck structure is pre-inserted, enabling us to insert the desired TCR gene like putting a cassette tape. Using this technology, the strategy of regenerated T cell therapy can expand its application.
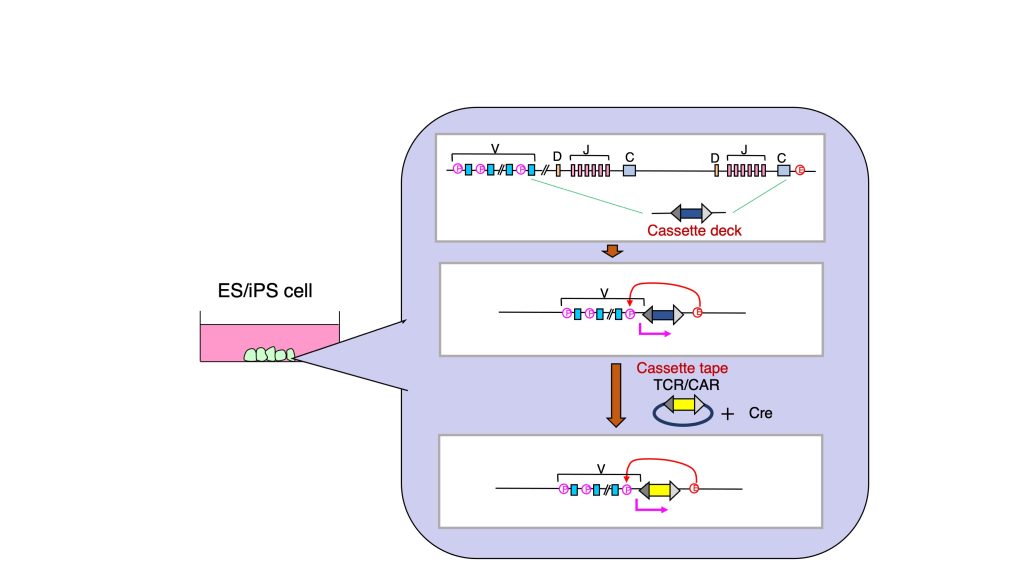
vi) Status
- July 2018: Initial application filed in Japan
- 2020: PCT application into the national phase in JP, US, EU, AU, and CN
- December 2023: Patented in JP
vii) Additional notes
- Rebirthel can license this patented technology unless the target antigen competes with Rebirthel or Rebirthel’s licensee.